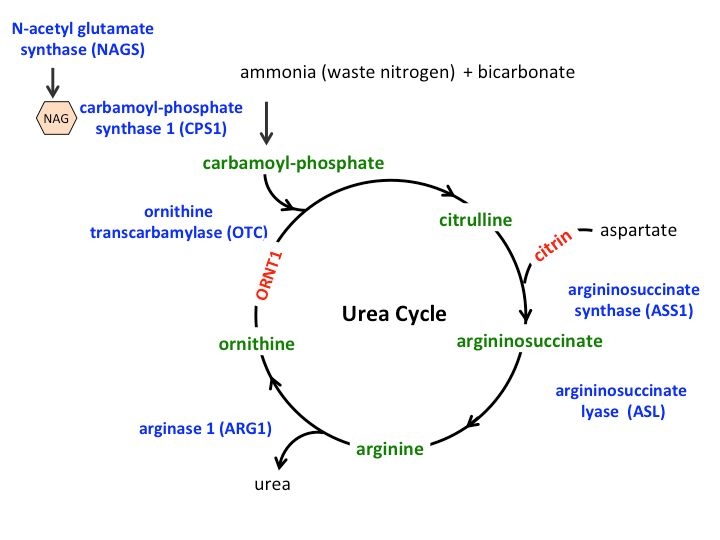
The global urea cycle disorders treatment market is estimated to be valued at US$587.52 Mn in 2024 and is expected to exhibit a CAGR of 5.3% over the forecast period 2024 to 2031, as highlighted in a new report published by Coherent Market Insights.
SWOT Analysis
Strength: Urea cycle disorder drugs are developing rapidly, providing more treatment options for patients. Newer drugs are more targeted and effective with fewer side effects. Government initiatives are raising awareness and screening for these rare genetic disorders.
Weakness: The small patient population makes drug development less profitable, so few companies actively research these conditions. Limited knowledge among medical professionals can delay diagnosis. High cost of medications presents difficulties for patients and healthcare systems.
Opportunity: Expanding newborn screening programs can help identify more cases early on. Advances in precision medicine may lead to personalized treatments. Partnerships between organizations can help pool resources to fund further research.
Threats: Restrictive regulations and lengthy approval processes hamper bringing new therapies to market quickly. Rising healthcare costs put pressure on budgets, risking less coverage or reimbursement for expensive treatments. Some disorders have no approved drugs yet.
Key Takeaways
The U.S. Urea Cycle Disorders Treatment Market Demand is expected to witness moderate growth over the forecast period driven by increasing research efforts. The small patient population has historically limited commercial incentives for drug development, but growing diagnosis and awareness of these rare genetic disorders are expanding the market. The global U.S. Urea Cycle Disorders Treatment Market is estimated to be valued at US$ 587.52 Mn in 2024 and is expected to exhibit a CAGR of 5.3% over the forecast period 2024 to 2031.
Regional analysis:
North America currently dominates the market and is expected to maintain its lead position over the forecast period due to strong research funding and healthcare infrastructure. Growing newborn screening programs are driving faster diagnosis rates. Europe is also a major regional market supported by universal healthcare coverage and initiatives to promote orphan drug development.
Key players:
Key players operating in the U.S. urea cycle disorders treatment market are Kawasumi Laboratories Inc., Blood Purification Technologies Inc., AWAK Technologies Pte. Ltd, Triomed AB, Asahi Kasei Medical Vo. Ltd, US Kidney Research Corporation, Merit Medical Systems, NIPRO Medical Corporation, Fresenius SE & Co. KGaA. The market remains fairly consolidated with a few leading pharmaceutical companies and biotech startups actively researching these rare conditions. Partnerships are common to share resources and accelerate drug development programs.
Explore more information on this topic, Please visit –
https://www.ukwebwire.com/u-s-urea-cycle-disorders-treatment-market-share-and-demand-analysis/
Explore more trending article related this topic –
https://www.ukwebwire.com/refurbished-electronics-market-trends-size-and-share-analysis/
https://whotimes.com/stock-music-market-an-ever-evolving-industry/