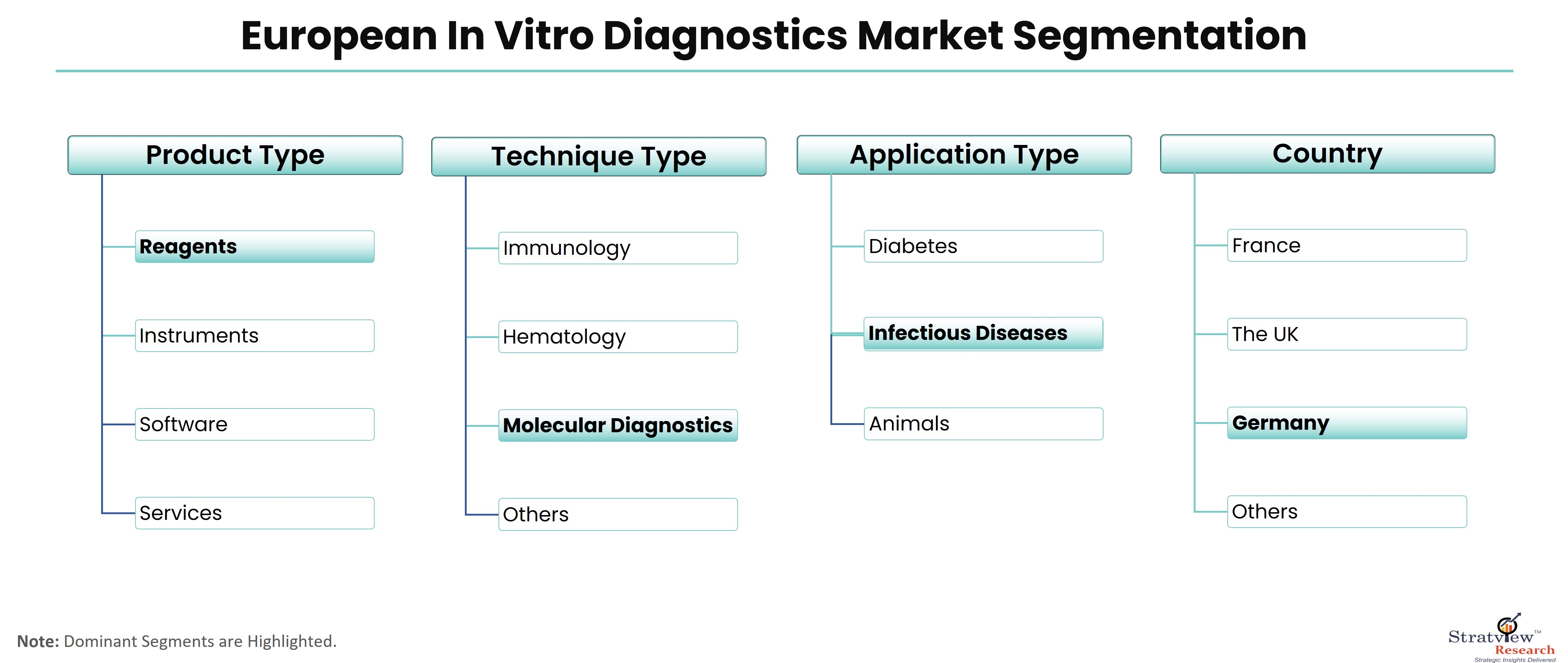
According to Stratview Research, the European in vitro diagnostics market was estimated at USD 15.29 billion in 2022 and is likely to grow at a CAGR of 5.08% during 2023-2028 to reach USD 20.66 billion in 2028.
In the complex tapestry of healthcare, challenges are inevitable, but so is the resilience and innovation that arise in response. At the forefront of overcoming health challenges in Europe stands the discipline of In Vitro Diagnostics (IVD), a critical component in the healthcare toolkit. In this exploration, we delve into the role of In Vitro Diagnostics in navigating health challenges, unraveling its significance in enhancing diagnostics, informing treatment strategies, and contributing to the resilience of European healthcare systems.
The Landscape of Health Challenges:
European healthcare faces a diverse array of challenges, ranging from the prevalence of chronic diseases to emerging infectious threats. These challenges are compounded by demographic shifts, an aging population, and the continuous need for effective strategies to manage both acute and chronic health conditions. In this landscape, In Vitro Diagnostics emerges as a key ally, providing the insights necessary to navigate through these complexities.
Precision in Diagnosis:
One of the foremost roles of In Vitro Diagnostics in navigating health challenges is its precision in diagnosis. Accurate and timely diagnosis is the linchpin for effective healthcare management. IVD technologies encompass a wide array of diagnostic tests, from routine blood screenings to sophisticated genetic analyses. The precision of these diagnostics allows healthcare professionals to identify diseases early, facilitating prompt interventions and personalized treatment plans.
Infectious Disease Management:
In the face of infectious disease challenges, In Vitro Diagnostics plays a pivotal role in identification, tracking, and containment. From rapid diagnostic tests for infectious agents to advanced molecular assays that characterize viral strains, IVD technologies are essential in responding swiftly to outbreaks. The ability to diagnose infectious diseases accurately is critical for implementing public health measures, contact tracing, and ultimately controlling the spread of pathogens.
Chronic Disease Monitoring:
The rising tide of chronic diseases, such as diabetes, cardiovascular conditions, and certain cancers, poses a persistent challenge to healthcare systems in Europe. In Vitro Diagnostics contributes significantly to the monitoring and management of chronic conditions. Regular testing allows healthcare providers to track disease progression, assess treatment efficacy, and make informed adjustments to patient care plans, promoting proactive and personalized healthcare.
Personalized Treatment Strategies:
Navigating health challenges in Europe involves moving beyond one-size-fits-all approaches to treatment. In Vitro Diagnostics facilitates the development of personalized treatment strategies. By analyzing individual biomarkers, genetic variations, and disease indicators, IVD technologies empower healthcare professionals to tailor treatments according to the unique characteristics of each patient. This personalized approach not only enhances treatment effectiveness but also minimizes the risk of adverse reactions.
Data-Driven Decision-Making:
In an era of data-driven healthcare, In Vitro Diagnostics provides the foundation for informed decision-making. The wealth of information generated through diagnostic tests offers insights into disease patterns, patient demographics, and the efficacy of healthcare interventions. Analyzing this data enables healthcare systems in Europe to strategize, allocate resources efficiently, and implement evidence-based practices to address prevailing health challenges.
Pandemic Preparedness and Response:
Recent global events have underscored the critical importance of preparedness and response to pandemics. In Vitro Diagnostics plays a crucial role in pandemic management by providing tools for rapid and widespread testing. From diagnosing infections to monitoring the efficacy of vaccination campaigns, IVD technologies contribute to an agile response in the face of emerging health challenges, helping to protect public health on a broad scale.
The Role of Point-of-Care Testing:
In navigating health challenges, the accessibility and speed of diagnostic results are paramount. Point-of-Care Testing (POCT) within In Vitro Diagnostics brings testing directly to the patient, minimizing turnaround times and facilitating rapid decision-making. This is particularly crucial in emergency situations, remote healthcare settings, and scenarios where immediate results can significantly impact patient outcomes.
Addressing Accessibility and Affordability:
While navigating health challenges, ensuring the accessibility and affordability of diagnostic services is a key consideration. In Vitro Diagnostics contributes to these goals by fostering innovation in testing technologies, streamlining workflows, and supporting initiatives for broader access to diagnostic services. Overcoming financial barriers and promoting inclusivity are essential aspects of leveraging IVD in the quest for equitable healthcare.
Conclusion: A Resilient Healthcare Foundation:
In conclusion, the role of In Vitro Diagnostics in navigating health challenges in Europe is akin to building a resilient foundation for healthcare systems. Its precision, adaptability, and contribution to personalized medicine position IVD technologies as indispensable tools in the hands of healthcare professionals. As Europe continues to face evolving health challenges, In Vitro Diagnostics stands as a beacon of innovation, offering insights that guide the way forward in the pursuit of optimal health outcomes for individuals and communities alike.